Zinc protects steel
One of zinc’s most important characteristics is its ability to protect steel against corrosion. The life and durability of steel are greatly improved when coated with zinc. No other material can provide such efficient and cost-effective protection for steel.
When left unprotected, steel will corrode in almost any exposed environment. Zinc coatings stop corrosion of steel in two ways – a physical barrier and electrochemical protection.
Barrier protection
Zinc coatings provide a continuous, impervious metallic barrier that does not allow moisture and oxygen to reach the steel. The metallic zinc surface reacts with the atmosphere to form a compact, adherent patina that is insoluble in rainwater. Typical coating thicknesses can range from 45μm to over 200μm.
Research over many years has shown that the life of this barrier protection is proportional to the zinc coating thickness. In other words, doubling the coating thickness will double the life of the coating.
Electrochemical protection
 Zinc also has the ability to galvanically protect steel. When bare steel is exposed to moisture, such as at a cut edge or damaged area, a galvanic cell is formed. The zinc around the point of damage corrodes in preference to the steel and forms corrosion products that precipitate on the steel surface and protect it. There is no sideways corrosion at points of damage.
Zinc also has the ability to galvanically protect steel. When bare steel is exposed to moisture, such as at a cut edge or damaged area, a galvanic cell is formed. The zinc around the point of damage corrodes in preference to the steel and forms corrosion products that precipitate on the steel surface and protect it. There is no sideways corrosion at points of damage.
Essentiality of zinc
All living organisms need zinc – it is an essential element. The amount of zinc present in nature varies widely, so living organisms have natural processes that regulate their uptake. Deficiency occurs when the amount of available zinc is insufficient to meet the needs of an organism. This occurs in both the environment and in human nutrition. It has been suggested that nearly half the world’s population is at risk from zinc deficiency and efforts are being made to boost the zinc intake of the world’s poorest children.
Zinc deficiency in agricultural soils is also common on all continents and leads to inefficiencies in crop and livestock production.
All living organisms need zinc – It is an essential element
Zinc enhances our memory and thinking by interacting with other chemicals to send messages to the sensory brain centre. Zinc can also reduce fatigue and mood swings.
Because zinc is used to generate cells, it is especially important during pregnancy, for the growing fetus whose cells are rapidly dividing.
In women, zinc can help treat menstrual problems and alleviate symptoms of premenstrual syndrome.
Zinc is vital for taste and smell, it is needed for renewal of skin cells and to keep our hair and nails healthy.
We use zinc in shampoo and sun-block products. In men, zinc protects the prostate gland and helps maintain sperm count and mobility.
Zinc helps keep us going… and enjoying healthy active lifestyles. Among all the vitamins and minerals, zinc shows the strongest effect on our all-important immune system.
Zinc has proven effective in fighting infections and can even reduce the duration and severity of the common cold.
Zinc is vital in activating growth in infants, children and teenagers.
Low-energy metal
Although only a small amount of zinc is used to conserve the embodied energy of steel, an important life cycle consideration for galvanizing is the energy used to produce zinc metal. The Swedish Environmental Protection Agency has compared the relative energy requirements of the common base metals and found that, with the exception of iron (the basis of steel), zinc uses the lowest energy on both a per unit weight and per unit volume basis. The energy used in electrolytic zinc production is approximately 7% for mining and mineral processing; 89% for electrolysis and 4% for casting.
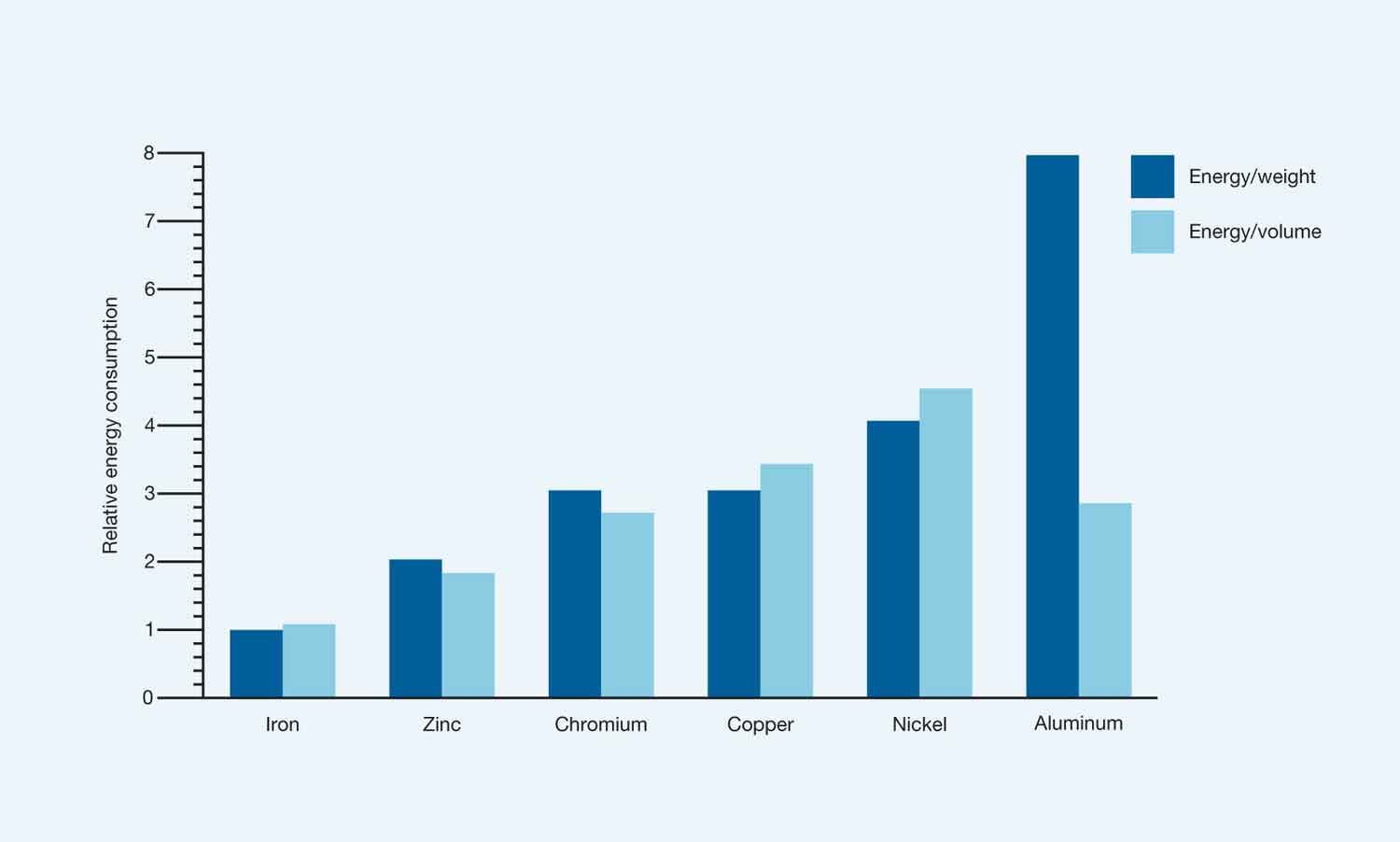
Energy use in primary (refined) production of various base metals in relation to weight and volume.
Energy use for iron/steel is set at one in both cases.
Zinc Recycling
 Zinc is an inherently recyclable non-ferrous metal and can be recycled indefinitely without any loss of physical or chemical properties. At present, approximately 70% of zinc comes from primary refining of zinc ores (including 10-15% from recycled sources) and about 30% comes directly from recycled zinc (representing 80% of the zinc available for recycling). The recycling level continues to increase as technology improves. The long life of zinc coated steel products in construction makes forecast of their emergence in waste streams difficult to model, hence more work will be required on this.
Zinc is an inherently recyclable non-ferrous metal and can be recycled indefinitely without any loss of physical or chemical properties. At present, approximately 70% of zinc comes from primary refining of zinc ores (including 10-15% from recycled sources) and about 30% comes directly from recycled zinc (representing 80% of the zinc available for recycling). The recycling level continues to increase as technology improves. The long life of zinc coated steel products in construction makes forecast of their emergence in waste streams difficult to model, hence more work will be required on this.
Zinc is an inherently recyclable non-ferrous metal and can be recycled indefinitely
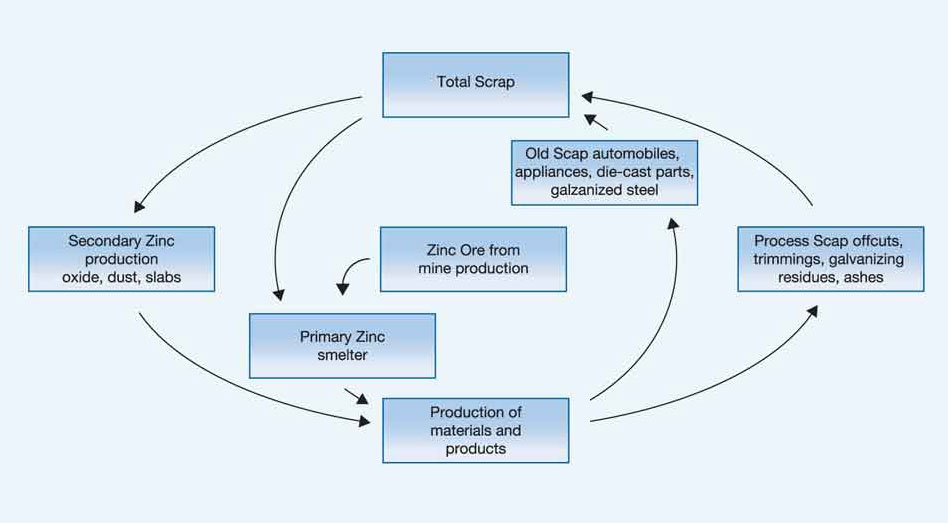
The zinc recycling circuit
Zinc Reserves
Zinc is the 27th most common element in the earth’s crust. The world is naturally abundant in zinc. Even a cubic mile of seawater is estimated to contain 1 tonne of zinc. It is estimated that the first mile of the earth’s crust under land contains 224,000,000 million tonnes of zinc, with a further 15 million tonnes in the seabed. Such estimates, however, take no account of whether or not it is economic, or environmentally acceptable, to exploit these resources.
Reserves of zinc – like those of any natural resource – are not a fixed amount stored in nature. Reserves are determined by geology and the interaction of economics, technology and politics. The term Reserves denotes the portion of resources that has been mapped and measured and which may be used, now or in the future.
Thus, Reserves reflect the state of knowledge, technology and the value of zinc at a given time. These natural resources are increasingly augmented by the supply of recycled zinc.
Proven Reserves of zinc have increased significantly since the 1950s, as large new ore bodies have been discovered in many areas of the world.
The sustainability of zinc ore supplies cannot therefore be judged simply by extrapolating the combined mine life of today’s zinc mines. Despite increasing consumption of zinc from 1995-2005, the world’s zinc reserves substantially increased over that same period, as shown in the table.
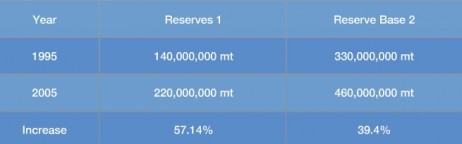
(Source: U.S. Geological Survey,)
1. Reserves are defined as, “That part of the reserve base which could be economically extracted or produced at the time of determination.”
2. Reserve base is defined as, “That part of an identified resource that meets specified minimum physical and chemical criteria related to current mining and production practices, including those for grade, quality, thickness, and depth.
Zinc production
80% of zinc mines are underground, 8% are of the open pit type and the remainder are a combination of both. Rarely is the ore, as mined, rich enough to be used directly by smelters; it needs to be concentrated.
Zinc ores contain 5-15% zinc. To concentrate the ore it is first crushed and then ground to enable optimal separation from the other minerals.
 Typically, a zinc concentrate contains about 55% of zinc, usually in the form of zinc sulphide. Zinc concentration is usually done at the mine site to keep transport costs to smelters as low as possible.
Typically, a zinc concentrate contains about 55% of zinc, usually in the form of zinc sulphide. Zinc concentration is usually done at the mine site to keep transport costs to smelters as low as possible.
Zinc concentrates are then roasted or sintered to convert zinc sulphide to zinc oxide. Zinc oxides are then processed in either pyrometallurgical, or more commonly, hydrometallurgical processes to produce zinc metal. The most common products are High Grade Zinc (99.95%) and Special High Grade Zinc (99.99%).
The zinc production industry’s commitment to sustainable development
In addition to the adoption of their Sustainability Charter in 2001, members of the International Zinc Association (IZA) defined an action plan to bring the zinc industry’s activities into harmony with the principles of sustainability.
Key elements of IZA’s sustainability strategy include:
• assessing future trends and developing zinc sustainability indicators
• developing and communicating a full understanding of the impact of zinc on the environment and its essential contribution to human health and eco-systems, based on a sound scientific risk assessment appropriate for zinc
• ensuring efficient use of resources to produce and recycle zinc
• reducing the energy intensity of all processes along the value-chain
• controlling emissions of zinc from point and diffuse sources
• producing according to appropriate social and environmental standards worldwide
• developing an integrated product policy throughout zinc’s life cycle
More information on the implementation of IZA’s sustainability strategy can be found at www.zinc.org/sustainability
Zinc in nature
Zinc, like all metals, is a natural component of the earth’s crust and an inherent part of our environment. Zinc is present not only in rock and soil, but also in air, water and the biosphere – plants, animals and humans. Zinc is constantly being transported by nature, a process called natural cycling. Rain, snow, ice, sun and wind erode zinc-containing rocks and soil. Wind and water carry minute amounts of zinc to lakes, rivers and the sea, where it collects as sediment or is transported further.
Natural phenomena such as:
• volcanic eruptions
• forest fires
• dust storms
• sea spray
all contribute to the continuous cycling of zinc through nature. During the course of evolution, all living organisms have adapted to the zinc in their environment and used it for specific metabolic processes. The amount of zinc present in the natural environment varies from place to place and from season to season. For example, the amount of zinc in the earth’s crust ranges between 10-300 milligrams per kilogramme, and zinc in rivers varies from less than 10 micrograms per litre to over 200 micrograms. Similarly, falling leaves in autumn lead to a seasonal increase in zinc levels in soil and water.
Every year an average sized Swedish river transports over ten tonnes of metals to the sea due to natural weathering and leaching from bedrock.

Zinc in the environment
Although zinc is well-recognised for its positive effects for humans and ecosystems, it is also important to avoid very high concentrations in the environment. Industrial emissions of zinc have been steadily falling over past decades.
Where locally high zinc concentrations may occur, for example in highly mineralised areas, nature has a remarkable ability to adapt. Nature also has mechanisms to bind zinc to reduce its so-called bioavailability. Bioavailability has been defined as “the amount or concentration of a chemical (metal) that can be absorbed by an organism thereby creating the potential for toxicity or the necessary concentration for survival” (Parametrix 1995). It is, however, not simply a function of the chemical form of the substance. Rather, it is largely influenced by the characteristics of the receiving environment. Hence, factors such as water hardness and pH have to be taken into account.
It is these bioavailability effects that explain why the apparently high soil zinc concentrations around large galvanized structures, such as electricity transmission towers, do not produce the toxic effects that may be predicted in the laboratory.
These factors have long been recognized as important, but there was insufficient scientific knowledge to allow a quantitative prediction of zinc’s bioavailability in a given set of conditions.
To address this, the galvanizing industry has contributed to extensive research to develop clear predictive models to quantify zinc bioavailability in waters, sediments and soils.
 There have been specific studies of contamination of soil and water from corrosion of galvanized products in the outside environment. Even in locations where many sources of zinc exist, such as at roadsides (where zinc can arise from tyre debris, lubricants, road wear and corrosion), these studies have shown that these releases do not give rise to adverse effects.
There have been specific studies of contamination of soil and water from corrosion of galvanized products in the outside environment. Even in locations where many sources of zinc exist, such as at roadsides (where zinc can arise from tyre debris, lubricants, road wear and corrosion), these studies have shown that these releases do not give rise to adverse effects.
The Division of Corrosion Science at the Royal Institute of Technology (KTH) in Stockholm has been studying the environmental impact of zinc, copper and stainless steel roofing materials. When it rains, the substances that are created through the corrosion of the roof surface are released. The amount of metal that can be released depends, on a number of different factors such as the amount of air pollution, the chemical composition and pH of the rain as well as the length and intensity of the rainfall.
The metals that exist in the run-off water leaving the edge of the roof consists mainly of free ions. Scientists at KTH found that once water had percolated through soil or had been in contact with concrete or limestone over 96% of its total metal content had been removed. The majority of metals bond very quickly on contact with the soil and the metals that remain in the water have a low bioavailability and therefore low potential for environmental effects.
Zinc Saves Kids
“Zinc Saves Kids” is an initiative of the International Zinc Association (IZA) to improve the survival, growth and development of undernourished children by funding UNICEF-supported zinc programs around the world.
IZA has committed to raise three million US$ over a three-year period from the global zinc community. The campaign will involve IZA member companies, zinc industry customer groups, suppliers and employees. The funds will be used to support zinc supplementation programs for children under the age of five who are most impacted by micronutrient deficiencies. 450,000 children under the age of five die annually due to zinc deficiency, and many who survive suffer lifelong repercussions from early childhood micronutrient deficiencies.

Zinc deficiency is a major health threat for children in low-income countries but it is one which can be readily addressed with cheap, simple and existing tools such as zinc supplements. A number of local zinc supplementation programs are already being implemented in some countries with excellent results, especially in the treatment and prevention of diarrhea. Diarrhea constitutes the second most common cause of child deaths worldwide. Two million children under the age of five die from diarrhea every year.
“Children are our greatest resource, we can’t allow that millions of them die every year, especially when zinc is part of the solution,” says Don Lindsay, President and CEO of Teck Resources Limited and Chairman of the International Zinc Association. “I strongly encourage our industry to help solve the global problem of malnutrition by donating to the Zinc Saves Kids campaign.

In addition to the ‘Zinc Saves Kids’ fundraising campaign, IZA is continuing to:
- Advance research on zinc’s benefits for human health and nutrition
- Raise public awareness of zinc as a problem of global public health significance that can be addressed in a cost-effective manner with existing means
- Seize every opportunity to urge governments, international and national development partners and other groups to support zinc programs
- Advocate and leverage additional funding from donor groups in support of zinc programs designed to improve child survival
- Promote the nutritional benefits of correcting zinc deficiency in crops


