Barrier protection
Galvanizing provides a barrier protection between all internal and external steel surfaces and their environment. Galvanizing is a term often wrongly used to describe zinc coatings in general. The diagram below illustrates how the different types of zinc coatings vary in terms of coating thickness. The life expectancy of a zinc coating is largely determined by its thickness. Thicker coatings give longer life. Hot dip galvanizing provides fabricated iron or steel products with maximum protection through a continuous, tough, metallurgically bonded coating of much greater thickness.
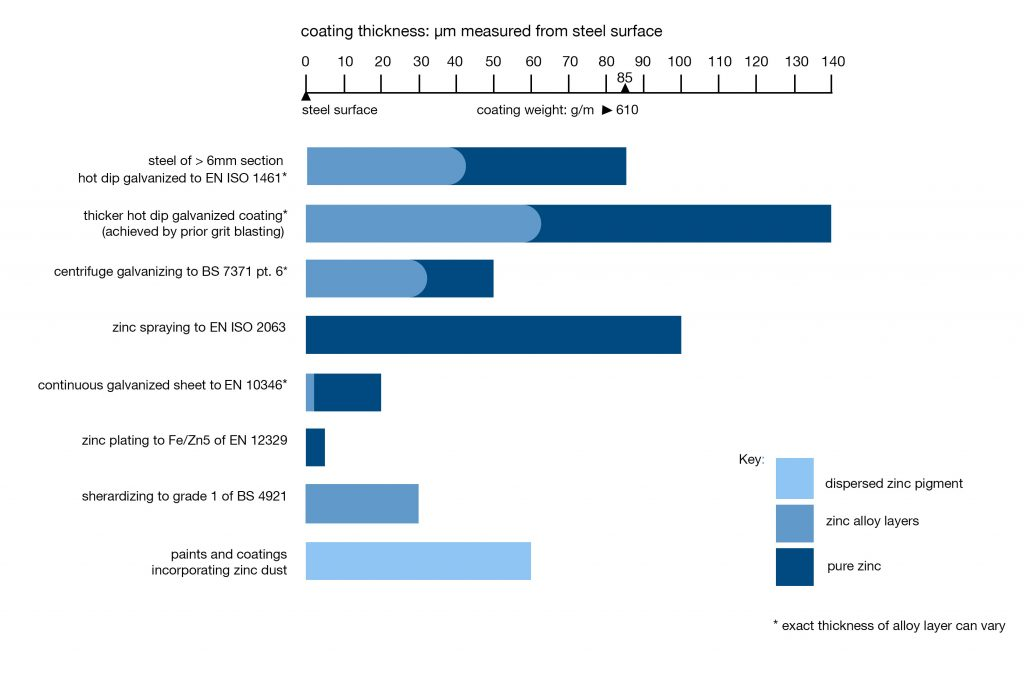
Zinc coatings compared in terms of coating thickness
Sacrificial protection
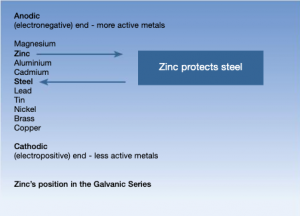 Zinc corrodes in preference to steel and sacrifices itself to protect the steel, hence hot dip galvanizing will provide this sacrificial protection.
Zinc corrodes in preference to steel and sacrifices itself to protect the steel, hence hot dip galvanizing will provide this sacrificial protection.
The corrosion products from the zinc are deposited on the steel resealing it from the atmosphere and therefore stopping corrosion.
With paint coatings, additional protection would have to be applied immediately after the damage occurred or the steel would rust with eventual breakdown of the whole coating as rust crept underneath the paint film.
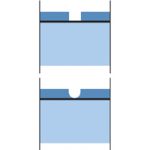
The diagram on the left illustrates how the sacrificial action of zinc protects the steel underneath. At the point where the galvanized steel suffers damage, a galvanic cell is formed. The zinc around the point of damage corrodes. Corrosion products precipitate on the steel surface and protect it. The steel is also protected because it is cathodic in relation to the zinc coating. (Read more on cathodic protection.)
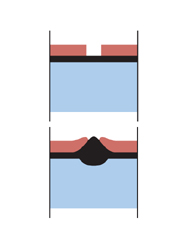
Let’s now think about what happens if you coat your steel with a more electro-positive metal such as Nickel, chromium and copper. As they fall below steel in the galvanic series, they give rise to more rapid corrosion at the point of damage than if the steel had been uncoated. The steel actually sacrifices itself in favour of the chrome, which is the opposite of what we are striving for when it comes to rust protection.
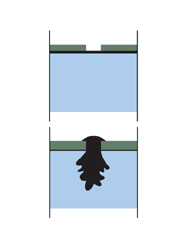
Nickel, chromium and copper give rise to more rapid corrosion at the point of damage than if the steel had been uncoated. The corrosion often takes the form of pitting, which can even go through the steel.
The unique nature of the galvanizing process provides a tough and abrasion resistant coating which means less site damage and speedy erection of structures. See galvanized coating intact after collision.
Galvanizers Association can provide detailed advice on compiling your specifications for galvanizing your steel, reviewing design details to maximise the benefits, as well as galvanizing standards. Learn more about the galvanizing standards.

