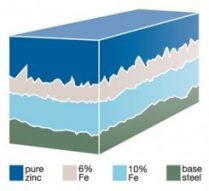
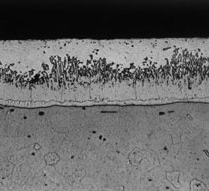
When the reaction between iron and zinc has virtually ceased and the article is taken out of the galvanizing bath complete with its outer coating of free zinc, the process is complete. In reality there is no demarcation between steel and zinc but a gradual transition through the series of alloy layers which provide the metallurgical bond.
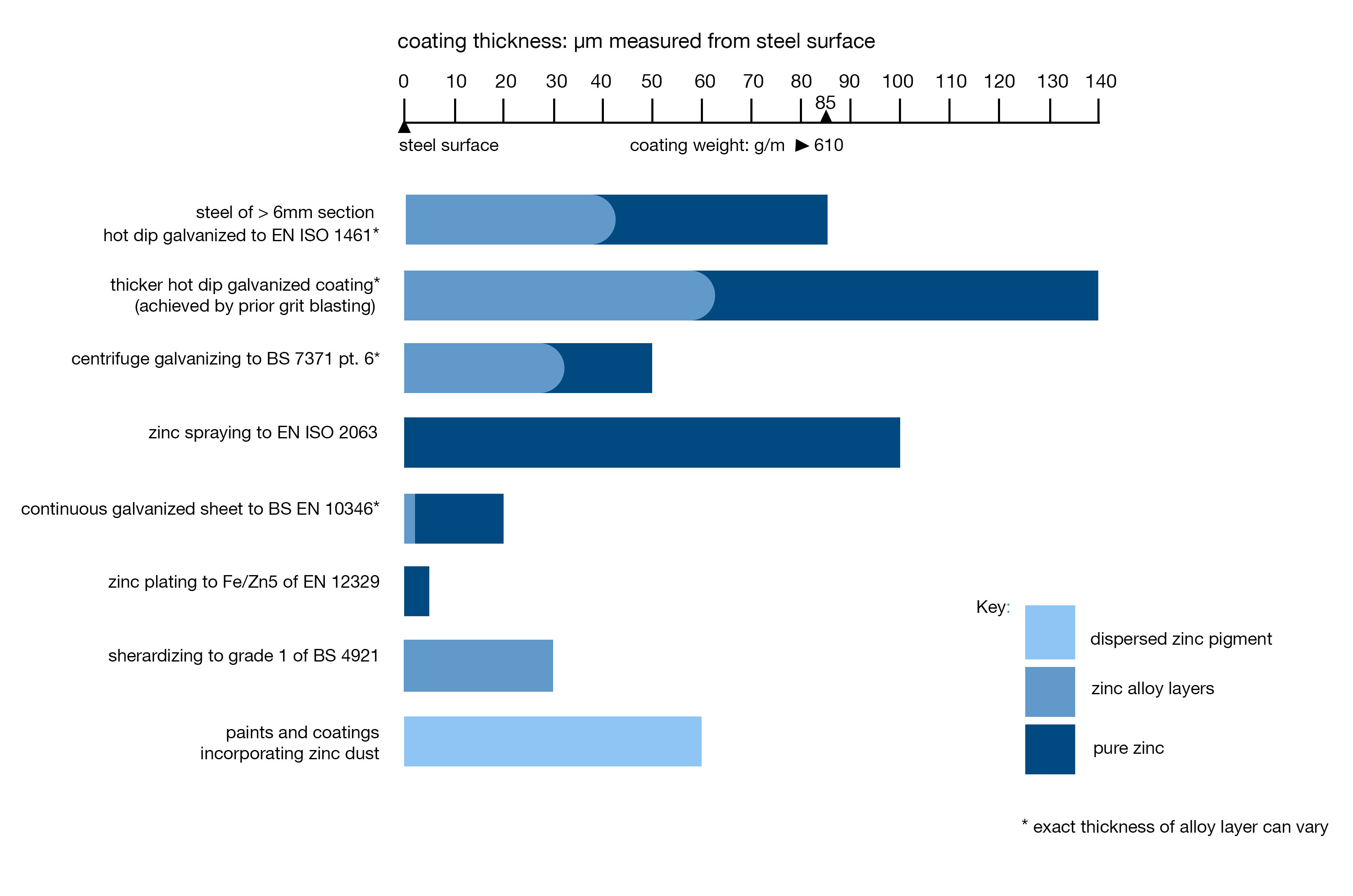
Coating thicknesses are normally determined by the steel thickness and are set out in EN ISO 1461. There are three exceptions to this rule, the first produces a slightly thinner coating, the other two increase it.
- Schematic section through a typical hot dip galvanized coating
- Microstructure of a typical hot dip galvanized coating
In reality there is no demarcation between steel and zinc, but a gradual transition through the series of alloy layers which provide the metallurgical bond.
Galvanised Coating Appearance
Considerations Before Galvanizing
Specifiers and end users often anticipate that batch hot dip galvanized steel will have a uniform bright colour and appearance, a view often formulated from having seen pregalvanized steel product (sheet) which is produced using a carefully controlled automated process.
In practice, when fabricated articles or individual steel sections are galvanized by the batch process to EN ISO 1461 some variation in coating appearance might occur. Read our blog post on ‘Why do I get variation in the colour of galvanized steel?’
The chemical composition and the condition of the steel which forms the base material are vitally important for successful hot dip galvanizing.
The formation of the zinc-iron alloy coating depends principally on the chemical composition of the steel that is galvanized. All common steels and irons can be hot dip galvanized, but steels with particular silicon contents may produce a very fast reaction between the iron and the zinc (read below about Galvanizing reactive steel).
For best results it is just as important for the customer to ensure careful preparation of the steel components as it is to ensure that the construction and design are suitable for galvanizing.
Galvanised Coating Thickness
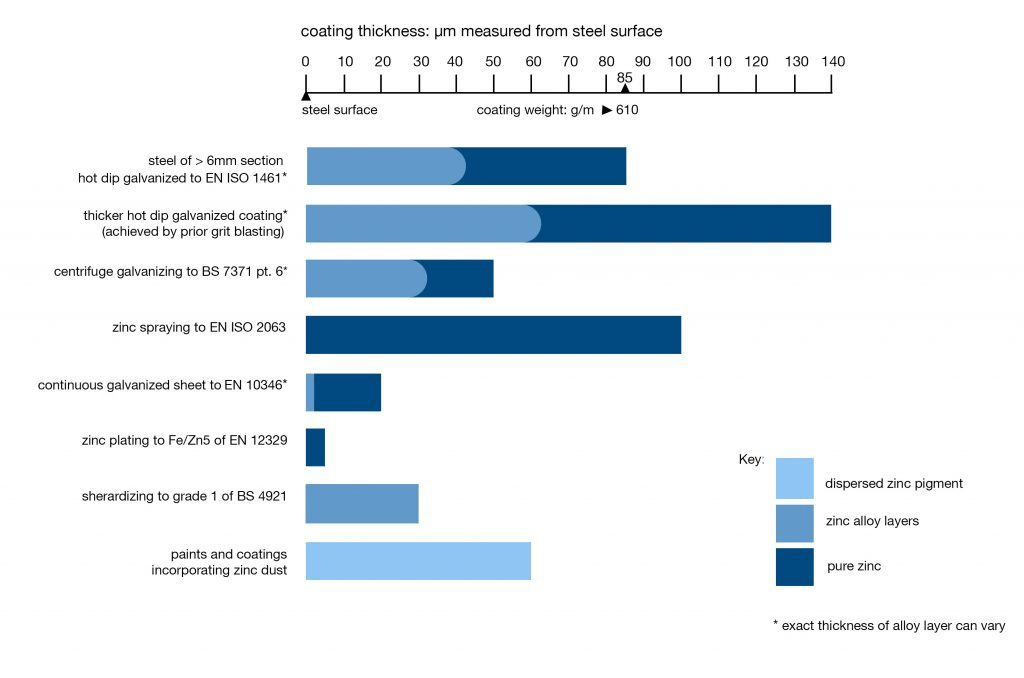
Galvanised Steel Coating Thickness versus other Zinc Coatings
How to Obtain Thicker Galvanised Coatings
Thicker coatings may be produced by one of the following:
- Centrifuged galvanized coating
This process, covered in EN ISO 1461, is used for galvanizing threaded components and other small parts. The parts, after preparation, are dipped in the molten zinc in a perforated basket. After the coating has formed this is centrifuged or spun at high speed, to throw off surplus zinc to ensure a clean profile. Minimum average coating weights for centrifuged work are identified in EN ISO 1461 and in 7371 Part 6. - Surface roughening
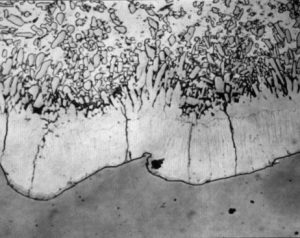
Microstructure of a thick coating obtained by grit blasting steel prior to galvanizing
This is the most common method of achieving thicker galvanised coatings. Grit blasting to Sa 2½ (ISO 7079) the steel surface prior to immersion, using chilled angular iron grit of size G24, roughens and increases the surface area of steel in contact with the molten zinc.
This generally increases the weight per unit area of a hot dip galvanized coating by up to 50%. Any steel article can be treated in this way, providing it is thick enough to withstand blasting. It may not be possible to grit blast the inside surface of hollow sections and fabrications, but these are almost always the areas least prone to corrosion.
Thicker galvanised coatings than those required by EN ISO 1461 should only be specified following consultation with the galvanizer or Galvanizers Association.
- Galvanizing reactive steel

Microstructure of the thick coating obtained using a silicon-rich steel
A thicker zinc coating will be obtained if the article to be galvanized is manufactured from a reactive steel. The constituents in steel that have the greatest influence on the iron/zinc reaction are silicon, which is frequently added to steel as a deoxidant during its production, and phosphorous.
Silicon changes the composition of the zinc-iron alloy layers so that they continue to grow with time and the rate of growth does not slow down as the layer becomes thicker. To a lesser degree, phosphorus exerts a similar influence on the formation of the coating.
When an article made from a reactive steel is removed from the zinc bath, a zinc layer adheres to the alloy layer as with any other steel article. However, the reaction rate in these steels can be so high that this pure zinc layer is transformed completely to zinc-iron alloy before the article has had time to cool.The result is a coating of equal or increased thickness that can be much darker in appearance. The change in appearance does not alter the corrosion resistance of the coating.